Nutritional Intervention during Muscle Injury Considering its Pathophysiology: Review Article
*Corresponding author: Luis Vergara-Gutiérrez luisvergara@medicinadeldeporte.cl
Cite this article
Vergara-Gutiérrez, L., Lizárraga-Dallo, A., & Pruna-Grive, R. (2020). Nutritional Intervention during Muscle Injury Considering its Pathophysiology: Review Article. Apunts. Educación Física y Deportes, 142, 8-20. https://doi.org/10.5672/apunts.2014-0983.es.(2020/4).142.02
Abstract
Injury generates physical, emotional and financial costs and so strategies have been sought to shorten the recovery period as much as possible. One underused tool is nutrition. A review of the scientific literature is presented by means of a search in four databases (Pubmed/MEDLINE, Epistemonikos, Embase and Sportdiscus) on the pathophysiology of muscle injury and its nutritional modulation. Local changes mediated by cells and inflammatory mediators and secondary changes in body composition are described. Published nutritional interventions include increasing dietary protein and adjusting carbohydrates in line with the athlete’s lower energy expenditure. There are no studies which have directly evaluated the use of supplements during injury, although there is mostly encouraging indirect evidence in sarcopenia and muscle recovery. Nutritional intervention during injury is essential to lessen the negative consequences of inactivity and should be indicated in an individualised way.
Introduction
The occurrence of injury is something which is inherent in the life of an athlete even though efforts are made to prevent it. It involves physical costs and also emotional and financial ones both for the player and also for the institution in the case of team sports (Wall et al., 2014). It is known that half of all sports injuries can be considered serious with an average of more than three weeks of inactivity without training or competing (Tipton, 2015). Any prolonged injury brings with it periods of rest resulting in loss of muscle mass, strength and function (Pierre et al., 2016) so any intervention to decrease the period of immobility will be significant. Furthermore, the return to competition may be further delayed by muscle atrophy and increased abdominal fat which may take several weeks to resolve. This accumulation of “unwanted” fat tissue is aggravated by a reduction in the local metabolic rate of the damaged tissue and by a decrease in the muscle’s sensitivity to insulin (Abadi et al., 2009; Pierre et al., 2016).
At present there are a number of treatments to foster and shorten recovery periods including cryotherapy, massage therapy, muscle stimulation and acupuncture (Dupuy et al., 2018). New treatments to shorten recovery times have also been investigated, such as platelet-rich plasma, monoclonal antibodies which inactivate inflammatory cytokines and local injection of growth factors among other invasive techniques. Many of these are expensive and present potential complications and adverse effects (Bachl et al., 2009; Mehrabani et al., 2019; Stöllberger & Finsterer, 2019).
One aspect that has been little studied, and at times underestimated, is the nutritional factor. Proper nutrition during the period of injury can help prevent the build-up of abdominal fat and might also optimise the tissue regeneration process and lessen muscle atrophy. It has been known for several years that nutrition can activate or inactivate the expression of our genome. A good example is leucine, an essential amino acid obtained from food which can activate the mTOR pathway by triggering protein synthesis, and hence it is used by many athletes to gain muscle mass (Duan et al., 2015; Li et al., 2011). If it were possible to plan a diet with the injured athlete featuring an appropriate intake of protein, foodstuffs with anti-inflammatory properties or ones which were able to modulate the immune response, the recovery period could be improved by achieving better quality tissue regeneration in the shortest possible time.
For centuries, many cultures have used foodstuffs with potential anti-inflammatory effects. However, the vast majority are not backed by sufficient scientific evidence to recommend them to athletes. Their usefulness, mechanism of action, appropriate dose and potential adverse effects are all unknown. It is therefore very important to have scientific proof of the highest possible quality in order to be able to indicate them with certainty as to their efficacy, thereby averting poor outcomes.
In recent years, studies have been conducted to test the immunomodulatory and anti-inflammatory effects of certain foodstuffs, most of which were performed in patients with sarcopenia or in muscle recovery after an eccentric exercise session (Beaudart et al., 2017; 2018; Chevalley et al., 2010; Colonetti et al., 2016; Cooke et al., 2010; Sousa et al., 2013). Their use in sports medicine, specifically for a sports injury, is an underdeveloped area yet with enormous potential as a future research strand bearing in mind how this intervention can reduce personal and financial costs. Consequently, the purpose of this review was to describe in detail the pathophysiology of muscle injury in each of its stages and then to present the most important aspects to be considered when conducting a nutritional intervention based on the scientific evidence available.
Methodology
This is a descriptive cross-sectional study of published articles by means of a narrative review. A search was conducted between February and July 2019 in four databases (Pubmed/MEDLINE, Epistemonikos, Embase and Sportdiscus) for scientific articles referring to the pathophysiology of muscle injury and its nutritional intervention during the recovery phase. Publications were included from the beginning of the indexation, giving priority to the search for systematic reviews and meta-analyses in the previous three years consistent with the classification of the quality of the evidence currently available. Publications not in Spanish or English and any not available in electronic databases were excluded. Initially a keyword search was performed with the Mesh tool including injury, athletic injuries, sports injury, nutrition, muscle regeneration, creatine, protein, whey protein, beta-hydroxy-beta-methylbutyrate, HMB, curcumin, vitamin D, Fatty Acids, Omega-3, Montmorency tart cherry and tart cherry. A further search was carried out using Boolean operators such as: “nutrition” OR “sports nutrition” AND “inj*”, “Athletic Inj*” OR “sports inj*” AND “Sports Nutritional Sciences”, “Athletic Inj*” AND “nutrition”, “muscle Inj*” AND “nutrition”, “protein” AND “athletic Inj*” OR “sports inj*”, “creatine” AND “athletic Inj*” OR “sports inj*”, “curcumin” AND “athletic Inj*” OR “sports inj*”, “Fatty Acids, Omega-3” AND “athletic Inj*” OR “sports inj*”, “vitamin D” AND “athletic Inj*” OR “sports inj*”, “tart cherry” OR “montmorency tart cherry” AND “athletic Inj*” OR “sports inj*”. A total of 21,498 articles were found. Duplicate studies and ones that did not match the objectives of the study were eliminated, resulting in 202 publications which were finally considered in the review. In the review articles found, the primary studies taken into account by the authors were used. If no studies were found whose direct purpose was sports injury, the best available indirect evidence was sought such as nutritional intervention in the recovery of muscle inflammation, sarcopenia in older adults or in patients hospitalised after surgery. A summary of the search strategy is shown in Figure 1.
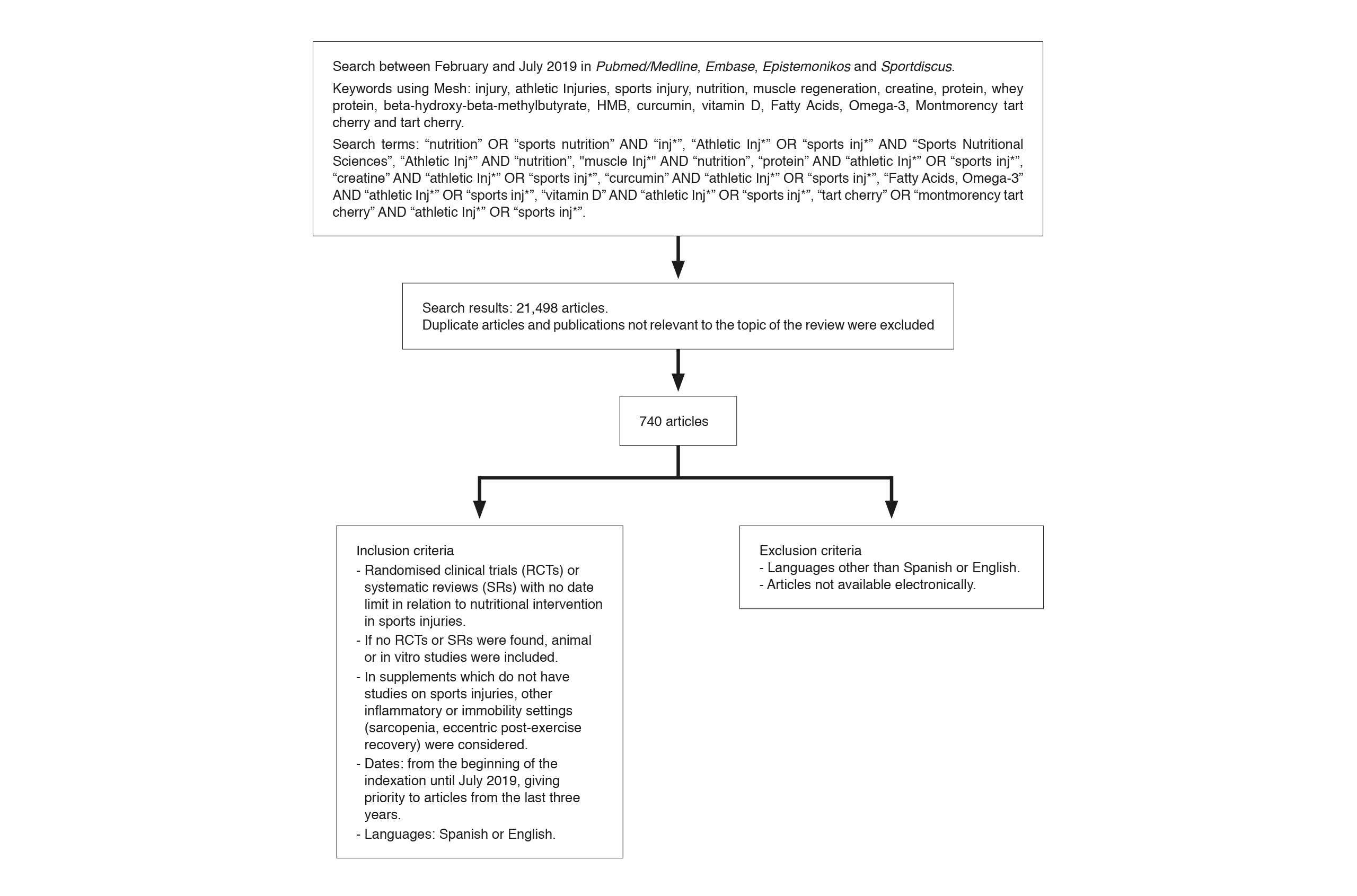
Summary of methodology and search for information
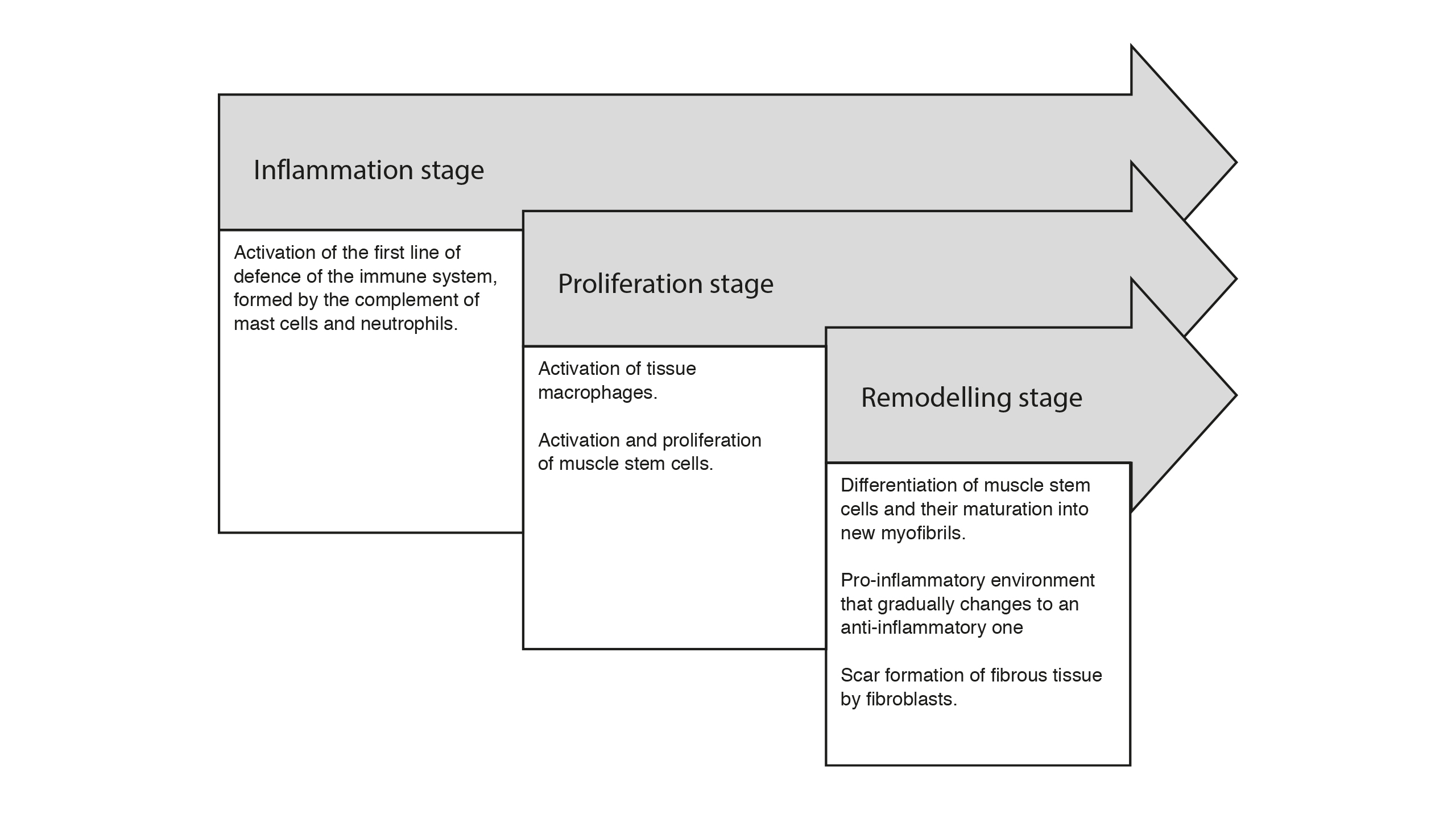
Diagram of the stages of muscle injury. They occur simultaneously and overlap with each other, which makes it difficult to delimit the beginning and end of each one
Results
No systematic reviews or randomised clinical trials concerning nutritional intervention during sports injury were found, making it impossible to conduct systematic reviews concerning the topic. Only five narrative reviews of the topic were found (Close et al., 2019; Medina et al., 2014; Tipton, 2010; 2015; Wall et al., 2014) describing in general terms potential nutritional interventions during the recovery period. In addition, 152 articles related to supplements were found which might be useful bearing in mind their possible mechanisms of action.
Local changes at the site of the muscle injury
Pathophysiology of muscle injury
Although there are several classifications, one of the most used divides the changes in the muscle injury into three stages which overlap, making it difficult to delimit the beginning and end of them since they occur simultaneously. The inflammation stage starts from the moment of injury until the seventh day with the involvement of neutrophils, macrophages and lymphocytes. The regeneration stage is considered to be approximately from the second to the seventh day with the activation and proliferation of the muscle stem cells which migrate and fuse to replace damaged muscle fibres. Finally, there is the remodelling stage between the fifth day and the fourth week when muscle fibres and the extracellular matrix are formed and regenerated (Cheng et al., 2008). These stages are summarised in Figure 2.
1) Inflammation stage
Immediately after the injury, an inflammatory response begins which can last anywhere from a few hours to several days depending on the type and severity of the damage (Tipton, 2010). The first stage of the recovery process is called inflammation and is mediated by activation of the complement, the mast cells and neutrophils, which are part of the immune system’s first line of defence.
It is characterised by the necrosis of the damaged fibres, which release their contents into the extracellular space including chemotactic factors which attract inflammatory cells.
Table 1
Summary of methodology and search for information
The complement system is the first sensor of muscle damage and is activated within seconds of injury. This activation allows neutrophils and macrophages to reach the site of damage (Frenette et al., 2000). In addition, the mast cells are also activated and their degranulation is one of the earliest responses of the innate immune system. Once activated, mast cells release inflammatory cytokines, such as tumour necrosis factor α (TNF-α), interleukin-1 (IL-1) and histamine, which in turn recruit more mast cells, neutrophils and other inflammatory cells (Yang et al., 2018). Finally, neutrophils are one of the most important inflammatory cells in the early stages after injury. Like mast cells, they are activated in skeletal muscle, releasing inflammatory cytokines such as TNF-α, IFN-γ and IL-1β. Their activation is very quick and they invade the damaged fibres within the first two hours of the injury. The number of neutrophils peaks between six and 24 hours after injury and declines rapidly by 72 to 96 hours (Arango et al., 2014). These neutrophils temporarily worsen the muscle damage and delay the next stage of cell regeneration by releasing IL-1 and IL-8 which induces the arrival of macrophages at the site of inflammation (Yang et al., 2018).
2) Second stage. Proliferation
It is characterised by the activation and proliferation of muscle stem cells associated with the appearance of macrophages and T cells. Muscle stem cells enable muscle cell regeneration. They are located within the basal lamina surrounding the myofibres, just between the muscle basement membrane and the basal lamina. They are present in all skeletal muscles and are associated with all types of muscle fibres, but not all of them have an equal distribution. For example, the percentage of muscle stem cells in adult soleus muscle (slow fibres) is two to three times larger than in the tibialis anterior muscle (fast fibres). Furthermore, their number decreases over the years which would explain why the regenerative capacity of skeletal muscle in response to damage decreases significantly with age (Järvinen et al., 2005).
Macrophages are produced in the bone marrow as monocytes, which are recruited by the action of neutrophils. They begin to be seen 24 hours after the injury, increasing two days later, and then decreasing rapidly until the fifth day (Tidball, 2005). Macrophages play a primary role in the regulation of muscle regeneration. They can be classified into two large groups: activated M1 macrophages, with an obviously inflammatory role, and M2 macrophages, also called “alternatively activated”, which are anti-inflammatory (Mantovani et al., 2004). M1 macrophages are the dominant ones during inflammation, removing the cellular debris generated by the trauma as a result of the release of cytokines such as TNF-α, IL-6 and IL-1β. TNF-α plays an important role in muscle regeneration. Laboratory studies with mice without this cytokine or with blockage of its receptor showed severe alterations in muscle regeneration (Chen et al., 2005). TNF-α can attract muscle stem cells to the site of injury and promote their proliferation by activating the nuclear transcription factor kappa β (NfKβ).
IL-6 is produced by multiple inflammatory cells, including macrophages and T-cells. It has been shown to stimulate myoblast migration, proliferation and differentiation. In animal models with overexpression of IL-6, activation of muscle stem cells was increased. In addition, IL-1β is mainly produced by macrophages, fostering the arrival of other macrophages and T cells and stimulating the production of IL-6 by muscle stem cells (Yang et al., 2018).
T cells are the largest cell population to be recruited in the second stage of inflammation. Both CD8+ and CD4+ cells are attracted to M1 macrophages, appearing at the site of injury on the third day and remaining until the tenth day (Cheng et al., 2008). Similar to macrophages, T cells synthesise a variety of growth factors and cytokines to modulate the inflammatory environment. Mice with T-cell deficiency show alteration in the regeneration process while adding them makes it possible to regain the regenerative process (Fu et al., 2015). Furthermore, the application of peptides secreted by human T cells accelerates the regeneration process, suggesting that these cytokines and growth factors secreted by T cells facilitate muscle regeneration (Yang et al., 2018).
3) Third stage. Remodelling
In this stage, muscle stem cells differentiate and mature into new myofibres. The pro-inflammatory environment gradually becomes an anti-inflammatory one, which is brought about by the change of macrophages from M1 (pro-inflammatory) to M2 (anti-inflammatory). M2 macrophages produce anti-inflammatory cytokines such as IL-4, IL-10 and IL-13. In addition, M2 macrophages are involved in the late stages of muscle regeneration. The absence of M2 macrophages leads to delayed muscle growth and inhibits muscle differentiation and regeneration.
Regulatory T cells are a type of T cell. Their number is low during the early stages of inflammation but increases sharply in later ones (Fu et al., 2015). These regulatory T cells secrete IL-10 and other cytokines to facilitate the conversion from M1 to M2 macrophages.
In lockstep, a scar of connective tissue is gradually formed at the site of the hematoma by the action of fibroblasts and which is designed to provide an anchor for the new myofibrils in formation. Fibroblasts are cells with a key role in tissue regeneration since they proliferate in close relationship with muscle stem cells. The absence of fibroblasts leads to early differentiation of the muscle stem cell, preventing the formation of myofibrils. Although fibroblasts are responsible for the formation of the fibrous scar, reciprocal stimulation with the muscle stem cells is very important to define whether a new myofibril will form or an unwanted collagen scar will develop.
A) Secondary changes during an injury
The period of inactivity associated with the inflammatory mediators during the injury has other consequences which if not addressed will further slow down the return to competition and the previously existing level of play.
A.1 Muscular atrophy
When an athlete has a major injury, in most cases there is an initial period of immobilisation or reduced physical activity called the “immobility or atrophy stage”, which can range from a few days to several months. Inactivity can result in significant losses of muscle mass and strength, which in turn also alter the structure and function of the tendon. Significant losses of muscle mass have been described with only five days of immobilisation. However, changes in gene expression within the first 48 hours of rest have also been studied. These changes in muscle mass are due to an imbalance between protein synthesis and protein degradation. Studies with isotopic markers show that both protein degradation and synthesis decrease after 14 days of strict bed rest, but synthesis is affected to a greater extent thus generating this negative protein balance (Tipton, 2015).
A.2 Loss of muscle metabolic flexibility
Metabolic flexibility is defined as the ability of the body to respond or adapt to energy or metabolic demands in a given situation. It has been linked to the generation of insulin resistance, obesity and type 2 diabetes mellitus (Goodpaster & Sparks, 2017). Mitochondrial oxidative function and metabolic flexibility are affected during the first weeks of rest as mitochondrial protein transcription lessens, signalling pathways related to mitochondrial biogenesis decrease and there is a significant fall in mitochondrial enzyme activity. Some of these changes are already visible 48 hours after the start of inactivity. This mitochondrial dysfunction leads to lower glucose transport by GLUT4 generating resistance to the action of insulin (Tipton, 2015), which also later on impacts the build-up of total body fat.
A.3 Anabolic resistance
During this rest period and associated with the synthesis of inflammatory mediators which also takes place at this stage, “anabolic resistance” is generated in which the response of muscle protein synthesis with the amino acids available is reduced. It is suggested that this anabolic resistance might be produced by slowed down digestion and absorption of amino acids, an alteration in muscle microvascular perfusion which would alter the incorporation of amino acids by the muscle and a blockage in intracellular anabolic molecular signalling (Glover et al., 2008).
A.4 Bone, tendon and ligament alterations
Immobilisation affects not only the muscle cell but also all the supporting tissue which is directly and indirectly involved with the muscle. During immobilisation, the synthesis of collagen by the tendon decreases, altering its mechanical properties which are critical to its functioning (Tipton, 2010). However, the changes which take place in the connective tissue surrounding the injury still need to be studied.
A.5 Deregulation of energy needs
It is obvious that energy requirements will be lower during the immobilisation stage as there is no training or competition. However, there are other changes in energy requirements which should also be borne in mind. The healing of the muscle injury itself will induce higher local energy demand due to the need to synthesise new proteins for recovery. This additional energy expenditure may increase by 15% to 50% depending on the degree of inflammation and severity, size and duration of the injury (Tipton, 2015). Furthermore, if the injury is in the legs, there will be problems with walking and sticks or braces will often be needed which can double the energy requirements for walking (Tipton, 2015). Consequently, the new energy requirements of the injured athlete need to be calculated individually depending on the type of injury and their daily activity.
A.6 Psychological and emotional response
When an injury occurs, the athlete experiences not only physical changes but also emotional ones. The recovery period will be a difficult time of anxiety and depression with the uncertainty of not knowing exactly what the rehabilitation process will be like and the potential implications for their performance when they return to competition. Dietary alterations are common, which may range from an increase in consumption of high-calorie foods to excessive restriction, both of which have consequences for their body composition. An athlete with depressive symptoms will adhere less to the medical team’s nutritional guidelines, so ideally it would be important to work with a sports psychologist to change the athlete’s perception of this period.
B) Nutritional intervention during injury
The first nutritional intervention described in the literature during the immobilisation period is to avoid nutritional deficits in vitamins, minerals and macronutrients. The macronutrient which would most impact recovery from injury would be protein in the diet, since it would not only alter myofibrillary protein synthesis but also have a direct effect on muscle metabolism. There are no studies which have determined the amount of protein needed to prevent muscle atrophy specifically during the dormant period of an athlete’s injury. Studies in men by Tipton et al. showed that providing high doses of protein (2.3 grams of protein per kilogram) decreased the loss of muscle mass in periods of negative energy balance compared to athletes who received 1 gram of protein per kilogram per day. This study was not conducted on injured athletes but rather during periods of weight loss (Tipton, 2015). It is known that providing 20 to 25 grams of protein in healthy active people in one dose maximises protein synthesis and anabolic resistance while the reduced physical activity suggests that a greater amount should be provided. This higher intake, which has not been described to date, should be distributed throughout the day. In the case of essential amino acids, there are also no papers on sports injuries.
The second intervention recommended in the literature is to adjust the athlete’s energy requirements. It is evident that the injured athlete will burn fewer calories per day due to their inactivity than one who trains normally. However, this adjustment is not so simple since other factors have to be taken into account. Scarring and protein synthesis generate an increase in energy expenditure which can rise by up to 50% depending on the type and severity of the injury and is estimated at up to 500 kcal in a man with significant muscle mass (Tipton, 2015). Furthermore, secondary expenditure on walking needs to be considered; an athlete who has to remain at complete rest in bed will not be the same as one who needs sticks which calls for greater energy expenditure. In spite of the above analysis, most often the injured athlete worsens their body composition due to their physical inactivity, increasing their fat mass both in the abdomen and in the limbs. It should be noted that not only is it important to adjust total daily calories but also the proportion of each of the macronutrients by decreasing carbohydrates and proportionally increasing protein intake.
Finally, the third intervention is the use of dietary supplements including whey protein, creatine, HMB and anti-inflammatories such as curcumin and tart cherry extract among others. To date, there are no studies using these supplements in sports injuries, but there is indirect evidence of their likely usefulness. Whey protein and curcumin have been shown to decrease inflammatory markers such as TNF-α, IL-1α and IL-1β (Derosa et al., 2016; Patel, 2015), so they could be used as immunomodulators in the early stages of muscle injury. An in vitro study showed that tart cherry can reduce COX-2 activity by 38.3%, which is equivalent to the effect of anti-inflammatory drugs such as ibuprofen or naproxen (Bell et al., 2013). Likewise, vitamin D receptors (VDR) have been found in in vitro studies on muscle stem cells which provide muscle regeneration after injury (Braga et al., 2017).

Table 2
Evidence for major nutritional supplements.
While there is indirect evidence for the use of dietary supplements to modulate muscle inflammation, the possible outcomes during an injury are unknown as the inflammatory process is acknowledged to be vital for proper tissue regeneration. For example, COX-2 through PGE2 plays a role in fibroblast proliferation and is a potent regulator of TGF-β1, which in turn is involved in collagen synthesis. Blocking TNF-α has also been shown to have negative effects on the recovery process, especially in its late stages (Sass et al., 2018; Tidball, 2005). Consequently, if it were possible to learn the right time to reduce inflammation without affecting the quality of the new tissue by using the appropriate supplements and/or foods, this process could be better modulated. An example would be to modify the activation of M1 macrophages towards an anti-inflammatory response mediated by M2 macrophages in chronic injuries with components present in the diet such as curcumin or tart cherry extract, which would make it possible to control the excessive inflammatory response which emerges in certain injuries.
Discussion
There are only a few published papers at present concerning nutritional interventions during the period of an injury. The vast majority of studies are conducted to promote muscle recovery or prevent osteopenia in older adults which means they are only indirectly applicable. More research on young athletes with sports injuries is needed to better apply the results.
A key stage in recovery from an injury is the inflammatory stage in which multiple inflammatory mediators, immune system cells and components of the extracellular matrix interact to initiate tissue repair. This stage could be modulated with anti-inflammatory foods to shorten the recovery period and thus reduce costs. However, it is known that the inflammatory process is vital for proper tissue regeneration. For example, blocking TNF-α has also been shown to have negative effects on the recovery process, especially in its later stages (Sass et al., 2018; Tidball, 2005). Consequently, if it were possible to identify the right time to reduce inflammation without affecting the quality of the new tissue by using the appropriate supplements and/or foods, this process could be better modulated. An example would be to modify the activation of M1 macrophages towards an anti-inflammatory response mediated by M2 macrophages in chronic injuries with components present in the diet such as curcumin or tart cherry extract, in which case the excessive inflammatory response which takes place in certain injuries might be controlled (Table 3).

Summary of the main inflammatory mediators present in recovery from a sports injury with their potential nutritional modulation based on current data
A very important point, and one which many athletes overlook, is that in order to get back to competition in the shortest time and at the highest possible level, not only does inflammation have to be modulated but also muscular atrophy and the accumulation of adipose tissue during the recovery period should be prevented as far as possible. The less atrophy and less increase in body fat there is, the faster recovery to the pre-injury level will be. It may take several weeks or even months to lose an excessive amount of fat and strengthen the muscles. This additional time is added after the athlete receives medical clearance to resume training and match play following a prolonged injury. To achieve these objectives, it is essential to adjust the energy requirements of the athlete by reducing their intake of carbohydrates and increasing protein in the diet to promote recovery. This adjustment needs to be individualised and tailored to each athlete. Severe calorie restriction may reduce protein synthesis by 20% to 30% (Tipton, 2015) which alters tissue regeneration and will exacerbate muscle atrophy. Regular monitoring is crucial to fine-tune the athlete’s diet as they increase their physical activity.
There are no studies directly evaluating the use of supplements in sports injuries. As noted above, it is difficult to draw valid conclusions given the varying doses, types of patients and exercise protocols performed. It is also hard to assess the impact of an isolated dietary component bearing in mind how challenging it is to control intake of other foodstuffs which may influence outcomes. In addition, the variables measured are often subjective and not easy to quantify such as the level of pain or the degree of injury healing. The above data call for careful analysis of the results of the available studies without drawing definitive conclusions and trying to apply them with criteria specific to each case until new scientific evidence for enhanced applicability emerges.
It is important to underline that the best nutritional intervention is to give priority to an appropriate diet by encouraging consumption of fruits and vegetables. If there is a need to add to daily protein intake, this can be done by increasing its consumption via food. In situations where it is difficult to provide large quantities through diet, as is the case with curcumin, supplements can be used to obtain suitable plasma levels more easily and conveniently.
Further studies will be needed in the coming years to investigate the relationship of food with immune and systemic inflammation processes. In the nutritional field, new research avenues should be opened on sports injuries to determine which foods or supplements stimulate the muscle stem cells to regenerate the damage, modulate the inflammatory response by fostering the anti-inflammatory activity of M2 macrophages, prevent the formation of a fibrous scar and avert muscle atrophy due to immobility as much as possible. Apart from their usefulness, the appropriate doses, the best time for their administration and their possible adverse effects also need to be known.
Conclusions
Considering both the pathophysiology and the multiple local and secondary changes after a sports injury, personalised nutritional intervention is called for in an athlete with a long recovery injury based on the recovery stage they have reached. Macronutrients should be tailored to the new energy requirements of a recovering athlete through a personalised diet. This would make it possible to control the inflammatory process, enhance the quality of muscle regeneration, shorten recovery times and minimise muscle atrophy and the accumulation of abdominal fat, thus adding to the work done by physiotherapists and rehabilitation therapists. To date, there is not enough scientific evidence on the use of food and supplements in sports injuries. More research designed specifically for these patients is needed to specify the foodstuffs and supplements which are beneficial, the right doses, the right time and the duration of treatment.
References
[1] Abadi, A., Glover, E. I., Isfort, R. J., Raha, S., Safdar, A., Yasuda, N., et al. (2009). Limb Immobilization Induces a Coordinate Down-Regulation of Mitochondrial and Other Metabolic Pathways in Men and Women. PLoS ONE, 4(8), e6518–14. https://doi.org/10.1371/journal.pone.0006518
[2] Arango Duque, G., & Descoteaux, A. (2014). Macrophage cytokines: involvement in immunity and infectious diseases. Frontiers in Immunology, 5(11), 491. https://doi.org/10.3389/fimmu.2014.00491
[3] Bachl, N., Derman, W., Engebretsen, L., Goldspink, G., Kinzlbauer, M., Tschan, H., et al. (2009). Therapeutic use of growth factors in the musculoskeletal system in sports-related injuries. The Journal of Sports Medicine and Physical Fitness, 49(4), 346–357.
[4] Beaudart, C., Dawson, A., Shaw, S. C., Harvey, N. C., Kanis, J. A., Binkley, N., et al. (2017). Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporosis International, 28(6), 1–17. https://doi.org/10.1007/s00198-017-3980-9
[5] Beaudart, C., Rabenda, V., Simmons, M., Geerinck, A., de Carvalho, I. A., Reginster, J. Y., et al. (2018). Effects of Protein, Essential Amino Acids, B-Hydroxy B-Methylbutyrate, Creatine, Dehydroepiandrosterone and Fatty Acid Supplementation on Muscle Mass, Muscle Strength and Physical Performance in Older People Aged 60 Years and Over. A Systematic Review of the Literature. The Journal of Nutrition, Health & Aging, 22(1), 117–130. https://doi.org/10.1007/s12603-017-0934-z
[6] Bell, P. G., McHugh, M. P., Stevenson, E., & Howatson, G. (2013). The role of cherries in exercise and health. Scandinavian Journal of Medicine & Science in Sports, 24(3), 477–490. https://doi.org/10.1111/sms.12085
[7] Bell, P., Stevenson, E., Davison, G., & Howatson, G. (2016). The Effects of Montmorency Tart Cherry Concentrate Supplementation on Recovery Following Prolonged, Intermittent Exercise. Nutrients, 8(7), 441–11. https://doi.org/10.3390/nu8070441
[8] Braga, M., Simmons, Z., Norris, K. C., Ferrini, M. G., & Artaza, J. N. (2017). Vitamin D induces myogenic differentiation in skeletal muscle derived stem cells. Endocrine Connections, 6(3), 139–150. https://doi.org/10.1530/EC-17-0008
[9] Capó, X., Martorell, M., Sureda, A., Tur, J. A., & Pons, A. (2016). Effects of dietary Docosahexaenoic, training and acute exercise on lipid mediators. Journal of the International Society of Sports Nutrition, 13(1), 1–13. https://doi.org/10.1186/s12970-016-0126-y
[10] Chen, S.-E., Gerken, E., Zhang, Y., Zhan, M., Mohan, R. K., Li, A. S., et al. (2005). Role of TNF-α signaling in regeneration of cardiotoxin-injured muscle. American Journal of Physiology-Cell Physiology, 289(5), C1179–C1187. https://doi.org/10.1152/ajpcell.00062.2005
[11] Cheng, M., Nguyen, M.-H., Fantuzzi, G., & Koh, T. J. (2008). Endogenous interferon-γ is required for efficient skeletal muscle regeneration. American Journal of Physiology-Cell Physiology, 294(5), C1183–C1191. https://doi.org/10.1152/ajpcell.00568.2007
[12] Chevalley, T., Hoffmeyer, P., Bonjour, J.-P., & Rizzoli, R. (2010). Early serum IGF-I response to oral protein supplements in elderly women with a recent hip fracture. Clinical Nutrition, 29(1), 78–83. https://doi.org/10.1016/j.clnu.2009.07.003
[13] Close, G. L., Sale, C., Baar, K., & Bermon, S. (2019). Nutrition for the Prevention and Treatment of Injuries in Track and Field Athletes. International Journal of Sport Nutrition and Exercise Metabolism, 29(2), 189–197. https://doi.org/10.1123/ijsnem.2018-0290
[14] Colonetti, T., Grande, A. J., Milton, K., Foster, C., Alexandre, M. C. M., Uggioni, M. L. R., & Rosa, M. I. D. (2016). Effects of whey protein supplement in the elderly submitted to resistance training: systematic review and meta-analysis. International Journal of Food Sciences and Nutrition, 68(3), 257–264. https://doi.org/10.1080/09637486.2016.1232702
[15] Cooke, M. B., Rybalka, E., Stathis, C. G., Cribb, P. J., & Hayes, A. (2010). Whey protein isolate attenuates strength decline after eccentrically-induced muscle damage in healthy individuals. Journal of the International Society of Sports Nutrition, 7(1), 30–9. https://doi.org/10.1186/1550-2783-7-30
[16] Da Boit, M., Hunter, A. M., & Gray, S. R. (2017). Fit with good fat? The role of n-3 polyunsaturated fatty acids on exercise performance. Metabolism: Clinical and Experimental, 66, 45–54. https://doi.org/10.1016/j.metabol.2016.10.007
[17] Daily, J. W., Yang, M., & Park, S. (2016). Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Journal of Medicinal Food, 19(8), 717–729. https://doi.org/10.1089/jmf.2016.3705
[18] Delfan, M., Ebrahim, K., Baesi, F., Mirakhori, Z., Ghalamfarsa, G., Bakhshaei, P., et al. (2015). The immunomodulatory effects of fish-oil supplementation in elite paddlers_ A pilot randomized double blind placebo-controlled trial. Prostaglandins Leukotrienes and Essential Fatty Acids, 99(C), 35–40. https://doi.org/10.1016/j.plefa.2015.04.011
[19] Derosa, G., Maffioli, P., Simental-Mendía, L. E., Bo, S., & Sahebkar, A. (2016). Effect of curcumin on circulating interleukin-6 concentrations: A systematic review and meta-analysis of randomized controlled trials. Pharmacological Research, 111, 394–404. https://doi.org/10.1016/j.phrs.2016.07.004
[20] Dreyer, H. C., Strycker, L. A., Senesac, H. A., Hocker, A. D., Smolkowski, K., Shah, S. N., & Jewett, B. A. (2013). Essential amino acid supplementation in patients following total knee arthroplasty. The Journal of Clinical Investigation, 123(11), 4654–4666. https://doi.org/10.1172/JCI70160
[21] Duan, Y., Li, F., Guo, Q., Wang, W., Zhang, L., Wen, C., et al. (2018). β-Hydroxy-β-methyl Butyrate Is More Potent Than Leucine in Inhibiting Starvation-Induced Protein Degradation in C2C12 Myotubes. Journal of Agricultural and Food Chemistry, 66(1), 170–176. https://doi.org/10.1021/acs.jafc.7b04841
[22] Duan, Y., Li, F., Li, Y., Tang, Y., Kong, X., Feng, Z., et al. (2015). The role of leucine and its metabolites in protein and energy metabolism. Amino Acids, 48(1), 41–51. https://doi.org/10.1007/s00726-015-2067-1
[23] Dupuy, O., Douzi, W., Theurot, D., Bosquet, L., & Dugué, B. (2018). An Evidence-Based Approach for Choosing Post-exercise Recovery Techniques to Reduce Markers of Muscle Damage, Soreness, Fatigue, and Inflammation: A Systematic Review With Meta-Analysis. Frontiers in Physiology, 9, 372–15. https://doi.org/10.3389/fphys.2018.00403
[24] Eijnde, B. O. ‘., Ursø, B., Richter, E. A., Greenhaff, P. L., & Hespel, P. (2001). Effect of Oral Creatine Supplementation on Human Muscle GLUT4 Protein Content After Immobilization. Diabetes…, 50(1), 18–23. https://doi.org/10.2337/diabetes.50.1.18
[25] Frenette, J., Cai, B., & Tidball, J. G. (2000). Complement Activation Promotes Muscle Inflammation during Modified Muscle Use. The American Journal of Pathology, 156(6), 2103–2110. https://doi.org/10.1016/S0002-9440(10)65081-X
[26] Fu, X., Xiao, J., Wei, Y., Li, S., Liu, Y., Yin, J., et al. (2015). Combination of inflammation-related cytokines promotes long-term muscle stem cell expansion. Nature Publishing Group, 25(6), 655–673. https://doi.org/10.1038/cr.2015.58
[27] Glover, E. I., Phillips, S. M., Oates, B. R., Tang, J. E., Tarnopolsky, M. A., Selby, A., et al. (2008). Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. The Journal of Physiology, 586(24), 6049–6061. https://doi.org/10.1113/jphysiol.2008.160333
[28] Goodpaster, B. H., & Sparks, L. M. (2017). Metabolic Flexibility in Health and Disease. Cell Metabolism, 25(5), 1027–1036. https://doi.org/10.1016/j.cmet.2017.04.015
[29] Henrotin, Y., Priem, F., & Mobasheri, A. (2013). Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. SpringerPlus, 2(1), 56. https://doi.org/10.1186/2193-1801-2-56
[30] Hespel, P., Op’t Eijnde, B., Van Leemputte, M., Ursø, B., Greenhaff, P. L., Labarque, V., et al. (2001). Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. The Journal of Physiology, 536(Pt 2), 625–633.
[31] Järvinen, T. A. H., Järvinen, T. L. N., Kääriäinen, M., Kalimo, H., & Järvinen, M. (2005). Muscle injuries: biology and treatment. The American Journal of Sports Medicine, 33(5), 745–764. https://doi.org/10.1177/0363546505274714
[32] Karimian, M. S., Matteo Pirro MD, P., Majeed, M., & Amirhossein Sahebkar PharmD, P. (2017). Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine and Growth Factor Reviews, 33, 55–63. doi:10.1016/j.cytogfr.2016.10.001
[33] Levers, K., Dalton, R., Galvan, E., O’Connor, A., Goodenough, C., Simbo, S., et al. (2016). Effects of powdered Montmorency tart cherry supplementation on acute endurance exercise performance in aerobically trained individuals. Journal of the International Society of Sports Nutrition, 1–24. https://doi.org/10.1186/s12970-016-0133-z
[34] Li, F., Yin, Y., Tan, B., Kong, X., & Wu, G. (2011). Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids, 41(5), 1185–1193. https://doi.org/10.1007/s00726-011-0983-2
[35] Liao, C.-D., Tsauo, J.-Y., Wu, Y.-T., Cheng, C.-P., Chen, H.-C., Huang, Y.-C., et al. (2017). Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. The American Journal of Clinical Nutrition, 106(4), 1078–1091. https://doi.org/10.3945/ajcn.116.143594
[36] Mantovani, A., Sica, A., Sozzani, S., Allavena, P., Vecchi, A., & Locati, M. (2004). The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology, 25(12), 677–686. https://doi.org/10.1016/j.it.2004.09.015
[37] Medina, D., Lizarraga, A., & Drobnick, F.2014. (s.d.). Injury prevention and nutrition in football. Sports Science Exchange, 27, 1–5.
[38] Mehrabani, D., Seghatchian, J., & Acker, J. P. (2019). Platelet rich plasma in treatment of musculoskeletal pathologies. Transfusion and Apheresis Science, 58(6), 102675–11. https://doi.org/10.1016/j.transci.2019.102675
[39] Minshull, C., Biant, L. C., Ralston, S. H., & Gleeson, N. (2015). A Systematic Review of the Role of Vitamin D on Neuromuscular Remodelling Following Exercise and Injury. Calcified Tissue International, 98(5), 426–437. https://doi.org/10.1007/s00223-015-0099-x
[40] Patel, S. (2015). Functional food relevance of whey protein: A review of recent findings and scopes ahead. Journal of Functional Foods, 19(PA), 308–319. https://doi.org/10.1016/j.jff.2015.09.040
[41] Pierre, N., Appriou, Z., Gratas-Delamarche, A., & Derbré, F. (2016). From physical inactivity to immobilization: Dissecting the role of oxidative stress in skeletal muscle insulin resistance and atrophy. Free Radical Biology and Medicine, 98, 197–207. https://doi.org/10.1016/j.freeradbiomed.2015.12.028
[42] Sahebkar, A. (2013). Are Curcuminoids Effective C-Reactive Protein-Lowering Agents in Clinical Practice? Evidence from a Meta-Analysis. Phytotherapy Research, 28(5), 633–642. https://doi.org/10.1002/ptr.5045
[43] Sahebkar, A., Cicero, A. F. G., Simental-Mendía, L. E., Aggarwal, B. B., & Gupta, S. C. (2016). Curcumin downregulates human tumor necrosis factor-α levels: A systematic review and meta-analysis ofrandomized controlled trials. Pharmacological Research, 107, 234–242. https://doi.org/10.1016/j.phrs.2016.03.026
[44] Sass, F. A., Fuchs, M., Pumberger, M., Geissler, S., Duda, G. N., Perka, C., & Schmidt-Bleek, K. (2018). Immunology Guides Skeletal Muscle Regeneration. International Journal of Molecular Sciences, 19(3). https://doi.org/10.3390/ijms19030835
[45] Schneider, A., Hossain, I., VanderMolen, J., & Nicol, K. (2017). Comparison of remicade to curcumin for the treatment of Crohn’s disease_ A systematic review, 33, 32–38. https://doi.org/10.1016/j.ctim.2017.06.002
[46] Sousa, M., Teixeira, V. H., & Soares, J. (2013). Dietary strategies to recover from exercise-induced muscle damage. International Journal of Food Sciences and Nutrition, 65(2), 151–163. https://doi.org/10.3109/09637486.2013.849662
[47] Stöllberger, C., & Finsterer, J. (2019). Side effects of whole-body electro-myo-stimulation. Wiener Medizinische Wochenschrift (1946), 169(7-8), 173–180. https://doi.org/10.1007/s10354-018-0655-x
[48] Tidball, J. G. (2005). Inflammatory processes in muscle injury and repair. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 288(2), R345–R353. https://doi.org/10.1152/ajpregu.00454.2004
[49] Tipton, K. D. (2010). Nutrition for acute exercise-induced injuries. Annals of Nutrition and Metabolism, 57 Suppl 2(s2), 43–53. https://doi.org/10.1159/000322703
[50] Tipton, K. D. (2015). Nutritional Support for Exercise-Induced Injuries. Sports Medicine, 45 Suppl 1(S1), S93–104. https://doi.org/10.1007/s40279-015-0398-4
[51] Wall, B. T., Morton, J. P., & Van Loon, L. J. C. (2014). Strategies to maintain skeletal muscle mass in the injured athlete: Nutritional considerations and exercise mimetics. European Journal of Sport Science, 15(1), 53–62. https://doi.org/10.1080/17461391.2014.936326
[52] Wu, H., Xia, Y., Jiang, J., Du, H., Guo, X., Liu, X., et al. (2015). Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: A systematic review and meta-analysis. Archives of Gerontology and Geriatrics, 61(2), 168–175. https://doi.org/10.1016/j.archger.2015.06.020
[53] Yang, W., & Hu, P. (2018). Skeletal muscle regeneration is modulated by inflammation. Journal of Orthopaedic Translation, 13, 1–8. https://doi.org/10.1016/j.jot.2018.01.002
[54] Yoneme, H., Hatakeyama, J., Danjo, A., Oida, H., Yoshinari, M., Aijima, R., et al. (2015). Milk basic protein supplementation enhances fracture healing in mice. Nutrition, 31(2), 399–405. https://doi.org/10.1016/j.nut.2014.08.008
ISSN: 2014-0983
Received: 10 February 2020
Accepted: 5 June 2020
Published: 1 October 2020
Editor: © Generalitat de Catalunya Departament de la Presidència Institut Nacional d’Educació Física de Catalunya (INEFC)
© Copyright Generalitat de Catalunya (INEFC). This article is available from url https://www.revista-apunts.com/. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en


