Assessment of body composition and bioimpedance in runners with Down syndrome: insights derived from a four-case study
*Corresponding author: Marta Carrasco-Marginet mcarrascom@gencat.cat
Cite this article
Cebrián-Ponce, A., Irurtia, A., Garnacho-Castaño, M. V., Espasa-Labrador, J., Castizo-Olier, J., Sarola, J. & Carrasco-Marginet, M. (2024). Assessment of body composition and bioimpedance in runners with Down syndrome: insights derived from a four-case study. Apunts Educación Física y Deportes, 158, 1-10. https://doi.org/10.5672/apunts.2014-0983.es.(2024/4).158.01
Abstract
Down syndrome (DS) is a genetic disorder that comes with a range of health challenges, including reduced cardiorespiratory fitness. For individuals with DS, accurate body composition assessment is challenging due to their unique body shapes. However, it is a critical component in early obesity detection and for developing targeted lifestyle interventions. The study involved four male DS runners who underwent anthropometric measurements and bioelectrical impedance vector analysis (classic and specific approaches) before and after completing the 14-kilometer race. Various equations were employed to estimate body composition. Additionally, somatotype analysis was conducted and bioelectrical changes evoked by the race were compared. Considerable variability in the body composition and race performance of DS individuals was revealed. Different equations for estimating fat mass yielded varying results (from 4.2 to 33.3%). Notably, the amount of fluids showed unique patterns among the participants. Participant 1 stood out with a remarkably high phase angle (9.8°), while the others had comparatively lower average values (4.5-6.3°). Bioelectrical impedance vector analysis indicated normal fluid loss during the race (T2 = 92.2; p < .0001). Intriguingly, Participant 1, who achieved the fastest race time, experienced the most significant fluid loss but displayed a greater intracellular water retention. This study underscores the importance of developing tailored body composition assessment methods for individuals with DS. Developing precise assessment tools will contribute to enhancing the well-being of this population as they pursue active lifestyles. These findings shed light on the intricate relationship between body composition, hydration, and performance in individuals with DS.
Introduction
Down syndrome (DS) is the most prevalent genetic disorder leading to intellectual disability worldwide (Franceschi et al., 2019). DS is associated with a host of health challenges that profoundly impact the quality of life and cardiorespiratory fitness of affected individuals (Seron et al., 2014). These challenges stem from factors such as cardiovascular diseases, muscle hypotonia, susceptibility to overweight/obesity, low bone mass, elevated body mass index (BMI), among others (Franceschi et al., 2019; Glasson et al., 2002). The predisposition to obesity in individuals with DS is exacerbated by their generally sedentary lifestyle (Florentino Neto et al., 2010), primarily due to the physical and physiological complexities associated with this condition. Nevertheless, exercise therapy has shown promise in normalizing autonomic function and mitigating the development of comorbidities (Cilhoroz et al., 2022). The utilization of appropriate body composition analysis methods may serve as a valuable tool for early obesity detection, facilitating the development of targeted lifestyle interventions aimed at preventing chronic diseases.
Numerous techniques exist for assessing body composition, including dual-energy X-ray absorptiometry (DXA), bioimpedance analysis (BIA), and kinanthropometry, among others. However, body composition significantly varies between individuals with and without DS (González-Agüero et al., 2017). This presents a challenge as many of the methods used to estimate fat mass percentage (%FM) are developed for the general population (Nickerson et al., 2023). This incongruity highlights the need for analyses tailored to the unique body shapes and morphologies of individuals with DS, as proposed by Rossato et al. (2018), based on the sum of four skinfolds (triceps, subscapular, biceps, and suprailiac), age, BMI, and gender. More recently, Nickerson et al. (2023) introduced a new equation based on mid-axilla and suprailium skinfolds, derived from a sample of 20 participants of varying ages and genders. The adoption of these specialized assessment methods could enhance the accuracy of body composition measurements in individuals with DS.
Bioelectrical impedance vector analysis (BIVA) emerges as an alternative approach for assessing body composition. It employs qualitative analysis by plotting participants within reference population ellipses using raw bioelectrical parameters, specifically resistance (R) and reactance (Xc), along with their derived components, impedance/vector length (Z) and phase angle (PhA) (Piccoli et al., 1994). BIVA offers a solution to the potential inaccuracies of predictive equations in populations with distinct characteristics by comparing participants’ vector positions to tolerance ellipses representing reference population values, requiring minimal elaborations. Two BIVA approaches exist, each tailored to standardizing bioelectrical parameters: classic BIVA, adjusting for height (R/H, Xc/H, Z/H) to account for conductor length and assess body fluids, and specific BIVA, further adjusting for height and cross-sectional areas of the arms, trunk, and legs (Rsp, Xcsp, Zsp) to mitigate the impact of body volume and estimate %FM (Campa et al., 2022a). Consequently, Z/H inversely correlates with total body water (Piccoli et al., 1994), while Zsp positively correlates with %FM (Toselli et al., 2020). PhA is considered an indicator of cellular health and cell membrane integrity, inversely linked to the extracellular/intracellular water (ECW/ICW) ratio, regardless of the BIVA approach (Marini et al., 2020). Notably, there exists a substantial lack of published research on BIVA in individuals with DS, with only a handful of conference posters available.
Hence, this preliminary study investigates the morphological characteristics of a sample of DS runners, employing anthropometric and BIVA (classic and specific). Furthermore, it aims to provide an initial comparison of bioelectrical values with the general population while also exploring potential bioelectrical changes induced by a 14-km race in individuals with intellectual disabilities.
Material and methods
Participants
This observational and descriptive study involved four male participants DS who were active runners. The participants took part in the “Volta a la Cerdanya Ultrafons®” 2013, a 14-kilometer race with an elevation gain of 489 meters. Inclusion criteria for the study were as follows: (a) participants aged 18 years or older with DS, and (b) the absence of injuries or clinical conditions at the time of the study. The competition was mixed-gender for individuals with and without disabilities. However, among the participants with disabilities, only males participated.
The study was conducted in accordance with the Declaration of Helsinki. All runners voluntarily participated and provided written informed consent before their participation. The research received prior approval from the Ethics Committee of the Catalan Sports Council (Approval No: 0099 S/690/2013).
Procedures
Anthropometric and bioelectrical measurements were conducted the morning before the race (PRE), under fasting conditions, and after participants had defecated and urinated. After completing the race, taking a shower, and towel drying, the same bioelectrical measurements were performed (POST). Throughout all measurements, participants were seated in a thermally neutral room and were not allowed to consume food or beverages. Immediately after completing the race, participants indicated their individual rate of perceived exertion (RPE) on a 10-point scale.
Anthropometry
Anthropometric measurements followed the standard criteria established by the International Society for the Advancement of Kinanthropometry (ISAK) (Stewart et al., 2011). The following measurements were recorded: body mass (BM), basic measurements (height, sitting height, and wingspan), nine skinfolds (triceps, subscapular, biceps, pectoral, iliac crest, supraspinal, abdominal, front thigh, and medial calf), seven girths (relaxed and flexed arm, waist, hip, mid-thigh, calf maximum, and ankle), and four breadths (humerus, wrist, femur, and ankle). Measurements were performed by an ISAK-accredited technician at Level 3 and recorded in millimeters on a modified ISAK proforma. Height was measured using a telescopic measuring rod (Seca 220®, Birmingham, UK; measuring range: 85-200 cm; accuracy: 1 mm), and BM was measured using a calibrated scale (Seca 710®, Birmingham, UK; capacity: 200 kg; accuracy: 50 g).
Skinfold thickness was measured on the right side of the body using a calibrated caliper (Holtain Limited, Sussex, UK; range: 0-80 mm, resolution: 0.20 mm, pressure: 10 g/mm2, accuracy: 99%). Girths were measured using a flexible anthropometric steel tape measure (Lufkin Executive®, Lufkin, TX, USA, accuracy: 1 mm). Breadths were obtained using a pachymeter (Holtain Limited, Sussex, UK; precision: 1 mm). Each measurement was conducted twice, and if the differences between skinfold measurements exceeded 5% or exceeded 1% for other measurements, a third measurement was performed. The final value for data analysis was the mean of two measurements or the median of three measurements, as appropriate.
Body mass index (BMI) was calculated as BM/H2 (kg/m2) and categorized as underweight (< 18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), or obese (≥ 30 kg/m2). Body adiposity index (BAI), based on the relationship between hip circumference and height, and relative fat mass (RFM), based on the relationship between waist circumference and height, were also determined. BAI categories included healthy (8-21%), overweight (21-26%), and obese (> 26%), while RFM was classified as fitness (14-17%), normal (18-24%), and obese (> 25%). Waist-to-hip ratio (WHtR) and waist-to-height ratio (WHR) cut-offs values of diagnostic overweight and obesity indices were placed at 0.56 and 0.87, respectively. Sum of six skinfolds (triceps, subscapular, supraspinal, abdominal, mid-thigh and calf maximum) and eight skinfolds (triceps, subscapular, biceps, iliac crest, supraspinal, abdominal, mid-thigh and calf maximum) were calculated, and equations by Durnin and Womersley (1974), Jackson and Pollock (1978), and Rossato et al. (2018) were applied to estimate %FM. The Siri equation (Siri, 1993) was applied to determine FM in the aforementioned equations in basis of body density.
Selected anthropometric measures were used to determine the somatotype components and draw the somatochart following Carter and Heath (1990), which defines the shape and composition of the human body through three numbers represented by endomorphy, mesomorphy, and ectomorphy.
Bioelectrical impedance analysis
R and Xc were measured using a BIA 101 Anniversary Sport Edition analyzer (Akern Srl, Florence, Italy), which emitted a 400 μA alternating sinusoidal current at 50 kHz. Prior to measurements, the device was calibrated with a known impedance circuit provided by the manufacturer (R = 383 ± 10 Ω, Xc = 45 ± 5 Ω). Bioelectrical variables were obtained by trained examiners, following the standard foot-to-hand electrode placement for tetrapolar measurements described by Kyle et al. (2004). Z was calculated as √(R2 + Xc2), and PhA was determined as tan-1 (Xc/R · 180°/π). For classic-BIVA, R, Xc, and Z were adjusted by height (R/H, Xc/H, Z/H), while specific-BIVA included adjustments for height and cross-sectional areas of the arm, trunk, and leg (Rsp, Xcsp, Zsp). The BIA equation proposed by Kotler (1996) was used to estimate %FM.
Statistical analysis
Descriptive data are presented as mean ± standard deviation. Selected anthropometric measures were used to determine somatotype components following the methods of Carter and Heath (1990), and participants were plotted in point graphs for both classic and specific approaches, with reference to a sample of healthy Italo-Spanish young adults (Ibáñez et al., 2015). Changes in bioelectrical values between PRE and POST were computed as delta percent values (∆%). RXc paired graphs and paired one-sample Hotelling’s T2 test were employed to assess differences between PRE and POST bioelectrical values. The significance level was set at p < .05. Data analysis was performed using SPSS (Chicago, IL, USA, ver. 21) and BIVA software (Piccoli & Pastori, 2002).
Results
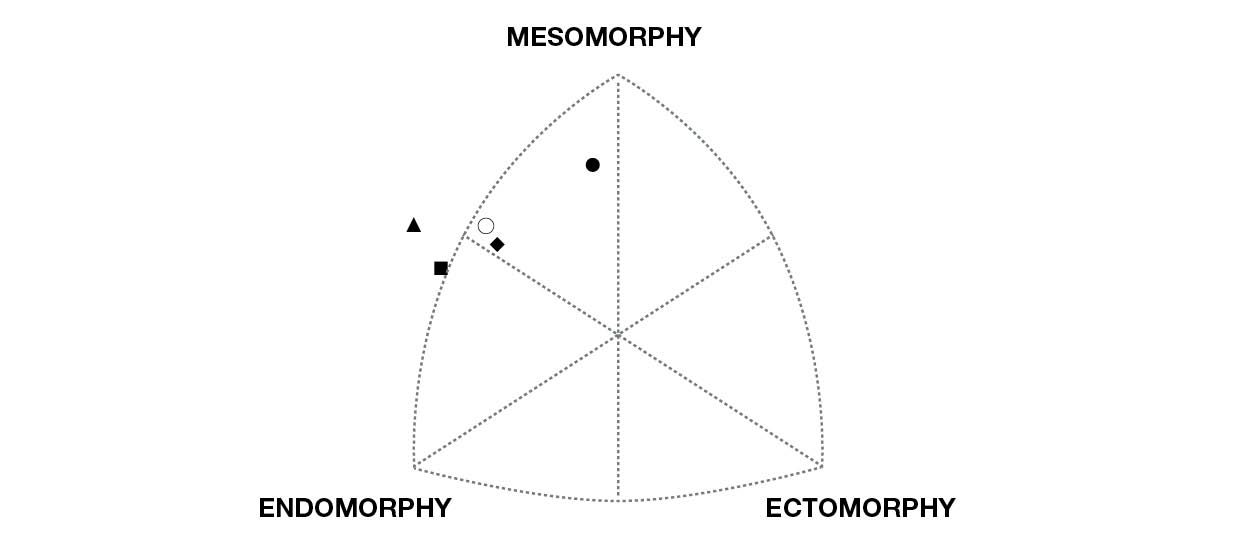
The comprehensive anthropometric profile of the four individuals with DS participating in the study is presented in Table 1, both individually and collectively. The age range among the participants is notably diverse, ranging from the youngest at 19 years old (Participant 1) to the eldest at 42.9 years old (Participant 4). In other basic measurements, there are largely similar values with minor differences observed. However, Participant 1 stands out with a substantially lower sum of skinfolds in both the six and eight skinfold measurements. Notably, there are variations in the calculated %FM across different equations. The most prevalent somatotype components among the participants are mesomorphy (observed in all participants) and endomorphy (except for Participant 1), as illustrated in Figure 1.
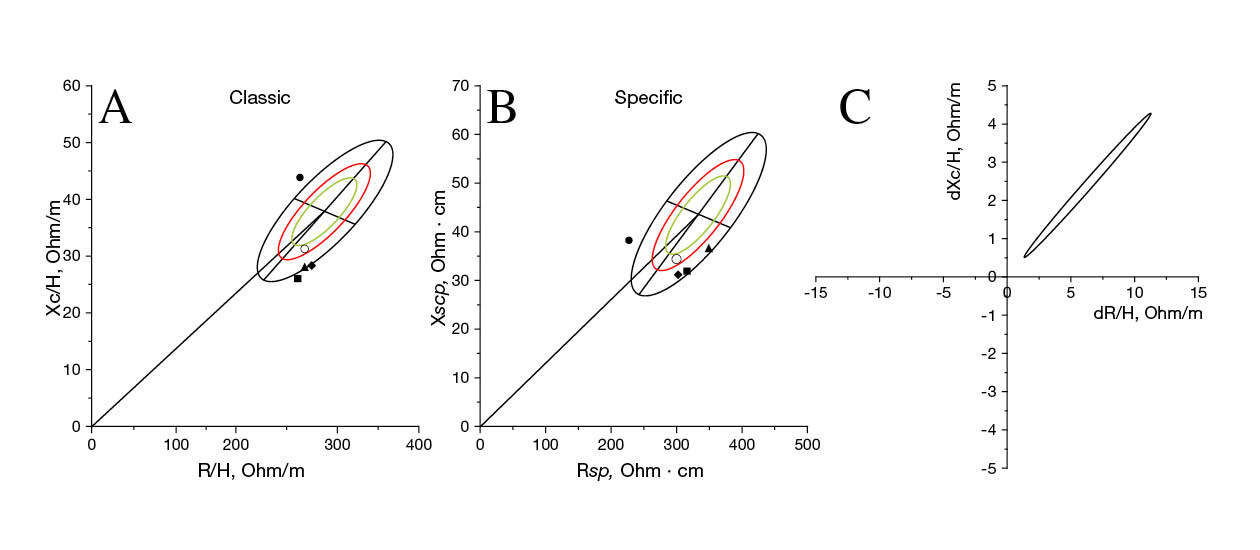
The race results, along with the PRE and POST bioelectrical values, are summarized in Table 2. A substantial discrepancy is evident in the race time of Participant 1 (99.5 minutes) compared to the remaining participants (170.8-208.5 minutes). None of the participants fall within the 95% classic tolerance ellipse or the 75% specific tolerance ellipse concerning the reference Italo-Spanish population, as indicated in both the classic (Figure 2A) and specific (Figure 2B) approaches.

Table 2
Race time, RPE and bioelectrical characteristics and changes produced by the 14-km race.
The high-intensity nature of the race (7.8 ± 0.5 out of 10-point RPE scale) is reflected in a decrease in body mass (BM) ranging from 1.0% to 1.7%, coupled with an upward trend in Z/H (2.1% to 3.3%) and PhA (3.6% to 6.3%) across all four participants. These changes are statistically significant, as demonstrated in Figure 2C (T2 = 92.2; p < .0001).
Discussion
The present study provides a comprehensive exploration of morphological profiles and bioelectrical changes in individuals with DS who participated in a demanding 14-km trail-race. Several critical insights and observations were derived from this research. First and foremost, it is imperative to acknowledge the significant variability in anthropometric and bioelectrical characteristics among the four participants with DS. Notably, Participant 1 exhibited unique attributes, including younger age, lower FM, and a higher PhA. Remarkably, Participant 1 also achieved the best race time by a considerable margin compared to the other participants. Secondly, the methods employed for estimating %FM demonstrated substantial disparities, underscoring the importance of using population-specific equations for individuals with DS. The bioelectrical values before the race fell outside the normal range when compared to individuals without disabilities, but all of them displayed a normal trend of fluid loss, as indicated by bioelectrical changes, which is a common response to endurance exercise.
Before delving into the analysis, it is essential to consider the age variability among the four participants, which varies from 19 to 42.9 years. This variation is particularly relevant given that DS is associated with premature aging, alongside functional and cognitive deterioration (Bittles et al., 2007). Therefore, it is unsurprising that both anthropometric and bioelectrical measurements differed among participants, especially in Participant 1 since is notably younger than the others.
Anthropometric assessment
Our participants exhibited a BMI of 26.8 ± 2.6 kg/m2 (Table 1), which closely aligns with the findings of a previous study involving male adolescents and young adults with DS (mean BMI: 26.1 ± 4.1 kg/m2) (Bandini et al., 2013). Interestingly, recent research has suggested an inverse relationship between BMI and cardiorespiratory fitness in adults with DS (Bittles et al., 2007). BMI is a simple measure of body composition that does not directly assess adiposity, but various equations based on it have been developed to estimate %FM. However, research by Esco et al. (2016) revealed that these equations are inadequate for individuals with DS, likely due to the distinct regional distribution of adipose tissue in this population (Fedewa et al., 2019). Consequently, specific equations tailored to individuals with DS are imperative for accurate assessments.
In our study, different equations yielded highly variable %FM results, with some equations producing unrealistic values. For instance, while BMI categorized Participants 1 and 4 as normal weight and Participants 2 and 3 as overweight, both BAI and RFM categorized all participants as “obese”. On the one hand, a recent study indicated that BAI may not be an adequate parameter because it overestimates %FM due to the low height of participants with DS (Fedewa et al., 2019; Rossato et al., 2017). On the other hand, RFM, although also height-based, appears to offer greater accuracy for both individuals with and without DS (Fedewa et al., 2019). Interestingly, Participant 1, who had the least subcutaneous tissue based on skinfold measurements, exhibited a higher BAI and RFM than Participant 4 and similar to Participant 3. Conversely, waist-to-height ratio (WHtR) and waist-to-hip ratio (WHR) did not classify any participants as overweight or obese, except for RFM in the case of Participant 3. These discrepancies among different assessment methods highlight the challenges of accurately determining %FM and associated health risks in individuals with DS.
As mentioned, existing equations for estimating FM based on skinfold anthropometric data for general population, such as those of Durnin and Womersley and Jackson and Pollock, are not suitable for individuals with DS. In these equations there is a notable difference between the 4 participants, with Participant 1 standing out again, since it presents 10.4% according to Durmin and Womersley and 4.2% according to Jackson and Pollock, demonstrating that these values are not correct, especially in the second equation. González-Agüero et al. (2017) developed a prediction equation for individuals with DS, specifically targeting adolescents aged 12 to 18, so this equation may not be applicable to adults. Therefore, Rossato et al. (2018) created a new equation for adults aged 18 to 47, so it fits our group. Nevertheless, this equation still resulted in significant variations among participants in our study, with Participant 1 showing markedly lower FM (6.6%) compared to the others (> 30%). Nickerson et al. (2023) in a recent study, and seeing the limitations of the current equations, proposed a new and more complete equation, which could not be replicated in this study because we lacked the anthropometric data required. Furthermore, a prediction equation based on bioelectrical values (Kotler et al., 1996) was used, and undoubtfully underestimated FM significantly (5.3-10.2%). This underestimation aligns with previous research findings (Esco et al., 2017), although a different device was used in our study.
Somatotype analysis (Figure 1) revealed that the mesomorphy component predominated in all participants, which is intriguing given that individuals with DS are typically characterized by lower muscle mass (Artioli et al., 2017). The endomorphic component was the second most prevalent, considerably higher than ectomorphy, which aligns more closely with expectations. The literature regarding the somatotype of participants with DS is almost null, with only one article by Bronks and Parker (1985) being identified. In such study, there was also a predominance of the endomorphic component, with 62% of the participants classified as mesomorphic-endomorphic. These results suggest an in-depth revision of this method for this specific population.
Bioelectrical assessment
In the classic point graph (Figure 2A), Participant 1 was positioned in the upper left quadrant of the reference population, while Participants 2, 3, and 4 were situated in the lower right quadrant, however, none of them fell within the 95% tolerance ellipse. The interpretation of these results suggests that the runners’ total body water, indicated by Z/H, was generally within the normal range, but intriguingly the PhA values exhibited noteworthy alterations. A higher PhA, which indicates better cell function due to its inverse relationship with the ECW/ICW ratio, is important for health and sports performance, as discussed by Sardinha (2018). Considering the reference percentile bioelectrical values for athletes developed by Campa et al. (2022b), Participant 1 PhA value (9.8°) surpasses the 95th percentile of the endurance athletes, which stands at 9.1°, signifying an unusually high PhA. In contrast, the PhA values of the remaining participants fell considerably below the 5th percentile of the reference values (6.3°). While these results align when comparing individuals with DS among themselves, comparing Participant 1 to the general population appears less logical.
In the specific point graph (Figure 2B), all participants appeared in the lower half of the tolerance ellipse, indicating lower levels of FM, with none falling within the 75% tolerance ellipse. Interpreting specific BIVA results requires caution due to the unique body volume characteristics of individuals with DS, where the normalization of this approach may render the values less suitable. It is crucial to consider that these are active individuals who may possess lower FM than their sedentary counterparts, although not necessarily less FM than individuals without DS.
During the race, participants experienced a modest BM decrease by 1.4 ± 0.3% (Table 2), accompanied by an increase in Z/H and PhA by 2.7 ± 0.6% and 5.8 ± 1.4%, respectively. These alterations reflect a notable loss of body fluids, primarily in the extracellular water compartment, as illustrated in Figure 2C. These changes fall within the expected range for endurance races, consistent with findings from previous studies (Castizo-Olier et al., 2018; Nescolarde et al., 2020). Notably, Participant 1, who achieved the fastest race time, exhibited the most significant increase in Z/H (3.3%) but the smallest rise in PhA (3.6%), suggesting a higher retention of intracellular water. This observation is noteworthy as intracellular water is recognized for its association with power and strength (Silva et al., 2014), potentially contributing to Participant 1’s superior performance.
Further research and study limitations
Physical exercise is known to positively influence cardiometabolic risk profiles, muscle strength, and aerobic capacity in both the general population and those with Down syndrome (Paul et al., 2019). Therefore, it is essential to conduct appropriate assessments to improve physical fitness and overall health, particularly in individuals with disabilities, where the rates of overweight and obesity are notably higher than in the general population (Pitchford et al., 2018). Future research should include larger, more varied samples across genders and ages. Until then, our study’s results are preliminary, particularly for active adult males with DS.
Some limitations should be acknowledged. There is a lack of detailed data on participants’ physical condition and dietary habits before and during the study. The small sample size of four participants, while a logistical challenge, limits the generalizability of the findings. Furthermore, the absence of a gold standard for determining FM prevents a definitive determination of which body composition method most accurately reflects reality. Future research should aim to address these limitations and provide a more comprehensive understanding of body composition and health in individuals with DS.
Conclusions
This study provides valuable insights into the morphological profiles and bioelectrical changes of individuals with DS following a demanding 14-km race. The findings emphasize the necessity of employing population-specific equations for accurate assessments of FM in individuals with DS and the importance of standardized approaches for evaluating health risks. Classic BIVA indicated a normal pattern of water loss due to the physical demands of the race.
As individuals with DS continue to engage in physical activities and sports, it is crucial to conduct appropriate assessments to enhance their physical fitness and overall health. Future research should expand on these findings and address the limitations identified in this study to provide a more comprehensive understanding of body composition and health in individuals with DS.
Acknowledgments
The authors wish to express their gratitude to all the volunteers. With the support of the National Institute of Physical Education of Catalonia (INEFC) of the Generalitat de Catalunya.
References
[1] Artioli, T. O., Witsmiszyn, E., Ferreira, A. B., & Pinto, C. F. (2017). Assessing Down syndrome BMI and body composition. International Medical Review on Down Syndrome, 21(2), 23-26. doi.org/10.1016/j.sdeng.2017.06.001
[2] Bandini, L. G., Fleming, R. K., Scampini, R., Gleason, J., & Must, A. (2013). Is body mass index (BMI) a useful measure of excess body fatness in adolescents and young adults with Down syndrome? Journal of Intellectual Disability Research, 57(11), 1050-1057. doi.org/10.1111/j.1365-2788.2012.01605.x
[3] Bittles, A. H., Bower, C., Hussain, R., & Glasson, E. J. (2007). The four ages of Down syndrome. European Journal of Public Health, 17(2), 221-225. doi.org/10.1093/eurpub/ckl103
[4] Bronks, R., & Parker, A. W. (1985). Anthropometric observation of adults with Down syndrome. American Journal of Mental Deficiency, 90(1), 110-113.
[5] Campa, F., Gobbo, L. A., Stagi, S., Cyrino, L. T., Toselli, S., Marini, E., & Coratella, G. (2022a). Bioelectrical impedance analysis versus reference methods in the assessment of body composition in athletes. European Journal of Applied Physiology, 122, 561-589. doi.org/10.1007/s00421-021-04879-y
[6] Campa, F., Thomas, D. M., Watts, K., Clark, N., Baller, D., Morin, T., Toselli, St., Koury, J. C., Melchiorri, G., Andreoli, A., Mascherini, G., Petri, C., Sardinha, L. B., & Silva, A. M. (2022b). Reference Percentiles for Bioelectrical Phase Angle in Athletes. Biology, 11(2), 264. doi.org/10.3390/biology11020264
[7] Carter, J. E. L., & Heath, B. H. (1990). Somatotyping: Development and Applications; Cambridge Studies in Biological and Evolutionary Anthropology. Cambridge University Press.
[8] Castizo-Olier, J., Carrasco-Marginet, M., Roy, A., Chaverri, D., Iglesias, X., Pérez-Chirinos, C., Rodríguez, F., & Irurtia, A. (2018). Bioelectrical impedance vector analysis (BIVA) and body mass changes in an ultra-endurance triathlon event. Journal of Sports Science and Medicine, 17(4), 571-579.
[9] Cilhoroz, B. T., Receno, C. N., Heffernan, K. S., & Deruisseau, L. R. (2022). Cardiovascular Physiology and Pathophysiology in Down Syndrome. Physiological Research, 71(1), 1-16. doi.org/10.33549/physiolres.934791
[10] Durnin, J. V, & Womersley, J. (1974). Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. The British Journal of Nutrition, 32(1), 77-97. doi.org/10.1079/bjn19740060
[11] Esco, M. R., Nickerson, B. S., Bicard, S. C., Russell, A. R., & Bishop, P. A. (2016). Agreement of BMI-based equations and DXA in determining body-fat percentage in adults with Down syndrome. Adapted Physical Activity Quarterly, 33(1), 89-96. doi.org/10.1123/APAQ.2014-0240
[12] Esco, M. R., Nickerson, B. S., & Russell, A. R. (2017). Comparison of bioelectrical impedance and DXA for measuring body composition among adults with Down syndrome. Disability and Health Journal, 10(4), 548-551. doi.org/10.1016/j.dhjo.2017.03.009
[13] Fedewa, M. V., Russell, A. R., Nickerson, B. S., Fedewa, M. P., Myrick, J. W., & Esco, M. R. (2019). Relative accuracy of body adiposity index and relative fat mass in participants with and without Down syndrome. European Journal of Clinical Nutrition, 73(8), 1117-1121. doi.org/10.1038/s41430-018-0351-3
[14] Florentino Neto, J., Pontes, L. M. de, & Fernandes Filho, J. (2010). Body compostion alterations resulting from weight training in subjects with Down Syndrome. Revista Brasileira de Medicina Do Esporte, 16(1), 09-12. doi.org/10.1590/s1517-86922010000100001
[15] Franceschi, C., Garagnani, P., Gensous, N., Bacalini, M. G., Conte, M., & Salvioli, S. (2019). Accelerated bio-cognitive aging in Down syndrome: State of the art and possible deceleration strategies. Aging Cell, 18(3), 1-11. doi.org/10.1111/acel.12903
[16] Glasson, E. J., Sullivan, S. G., Hussain, R., Petterson, B. A., Montgomery, P. D., & Bittles, A. H. (2002). The changing survival profile of people with Down’s syndrome: Implications for genetic counselling. Clinical Genetics, 62(5), 390-393. doi.org/10.1034/j.1399-0004.2002.620506.x
[17] González-Agüero, A., Matute-Llorente, Á., Gómez-Cabello, A., Vicente-Rodríguez, G., & Casajús, J. A. (2017). Percentage of body fat in adolescents with Down syndrome: Estimation from skinfolds. Disability and Health Journal, 10(1), 100-104. doi.org/10.1016/j.dhjo.2016.05.013
[18] Ibáñez, M. E., Mereu, E., Buffa, R., Gualdi-Russo, E., Zaccagni, L., Cossu, S., Rebato, E., & Marini, E. (2015). New Specific Bioelectrical Impedance Vector Reference Values for Assessing Body Composition in the Italian-Spanish Young Adult Population. American Journal of Human Biology, 27, 871-876. doi.org/10.1002/ajhb.22728
[19] Jackson, A. S., & Pollock, M. L. (1978). Generalized equations for predicting body density of men. British Journal of Nutrition, 40(3), 497-504. doi.org/10.1079/bjn19780152
[20] Kotler, D. P., Burastero, S., Wang, J., & Pierson Jr., N. R. (1996). Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: Effects of race, sex, and disease. American Journal of Clinical Nutrition, 64(SUPPL.), 489S-497S.
[21] Kyle, U. G., Bosaeus, I., De Lorenzo, A. D., Deurenberg, P., Elia, M., Gómez, J. M., Heitmann, B. L., Kent-Smith, L., Melchior, J. C., Pirlich, M., Scharfetter, H., Schols, A. M. W. J., & Pichard, C. (2004). Bioelectrical impedance analysis - Part I: Review of principles and methods. Clinical Nutrition, 23(5), 1226-1243. doi.org/10.1016/j.clnu.2004.06.004
[22] Marini, E., Campa, F., Buffa, R., Stagi, S., Matias, C. N., Toselli, S., Sardinha, L. B., & Silva, A. M. (2020). Phase angle and bioelectrical impedance vector analysis in the evaluation of body composition in athletes. Clinical Nutrition, 39(2), 447-454. doi.org/10.1016/j.clnu.2019.02.016
[23] Nescolarde, L., Roca, E., Bogónez-Franco, P., Hernández-Hermoso, J., Bayes-Genis, A., & Ara, J. (2020). Relationship Between Bioimpedance Vector Displacement and Renal Function After a Marathon in Non-elite Runners. Frontiers in Physiology, 11(May), 1-13. doi.org/10.3389/fphys.2020.00352
[24] Nickerson, B. S., Esco, M. R., & Schaefer, G. (2023). Evaluation of Skinfold Techniques in People with Down Syndrome: Development of a New Equation. International Journal of Environmental Research and Public Health, 20(10). doi.org/10.3390/ijerph20105831
[25] Paul, Y., Ellapen, T. J., Barnard, M., Hammill, H. V., & Swanepoel, M. (2019). The health benefits of exercise therapy for patients with Down syndrome: A systematic review. African Journal of Disability, 8. doi.org/10.4102/ajod.v8i0.576
[26] Piccoli, A., & Pastori, G. (2002). BIVA SOFTWARE. In University of Padova (pp. 1-17).
[27] Piccoli, A., Rossi, B., Pillon, L., & Bucciante, G. (1994). A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney International, 46(2), 534-539. doi.org/10.1038/ki.1994.305
[28] Pitchford, E. A., Adkins, C., Hasson, R. E., Hornyak, J. E., & Ulrich, D. A. (2018). Association between Physical Activity and Adiposity in Adolescents with Down Syndrome. Medicine and Science in Sports and Exercise, 50(4), 667-674. doi.org/10.1249/MSS.0000000000001502
[29] Rossato, M., Dellagrana, R. A., Da Costa, R. M., De Souza Bezerra, E., Otacílio Libardoni, J., & Rech, C. R. (2018). The Accuracy of Anthropometric Equations to Assess Body Fat in Adults with Down Syndrome. Journal of Applied Research in Intellectual Disabilities, 31(2), 193-199. doi.org/10.1111/jar.12290
[30] Rossato, M., Dellagrana, R. A., De Souza Bezerra, E., Da Costa, R. M., Dos Santos, J. O. L., Silva, D. A. S., & Diefenthaeler, F. (2017). Comparison of body adiposity index (BAI) and air displacement plethysmograph with estimations of % body fat in adults with Down’s syndrome. European Journal of Clinical Nutrition, 71(11), 1341-1344. doi.org/10.1038/ejcn.2017.18
[31] Sardinha, L. B. (2018). Physiology of exercise and phase angle: another look at BIA. European Journal of Clinical Nutrition, 72(9), 1323-1327. doi.org/10.1038/s41430-018-0215-x
[32] Seron, B. B., Silva, R. A. C., & Greguol, M. (2014). Effects of two programs of exercise on body composition of adolescents with Down syndrome. Revista Paulista de Pediatria, 32(1), 92-98. doi.org/10.1590/s0103-05822014000100015
[33] Silva, A. M., Matias, C. N., Santos, D. A., Rocha, P. M., Minderico, C. S., & Sardinha, L. B. (2014). Increases in intracellular water explain strength and power improvements over a season. International Journal of Sports Medicine, 35(13), 1101-1105. doi.org/10.1055/s-0034-1371839
[34] Siri, W. E. (1993). Body composition from fluid spaces and density: analysis of methods. 1961. Nutrition (Burbank, Los Angeles County, Calif.), 9(5), 480-491; discussion 480, 492.
[35] Stewart, A., Marfell-Jones, M., Olds, T., & de Ridder, H. (2011). International standards for anthropometric assessment. International Society for the Advancement of Kinanthropometry.
[36] Toselli, S., Marini, E., Latessa, P. M., Benedetti, L., & Campa, F. (2020). Maturity related differences in body composition assessed by classic and specific bioimpedance vector analysis among male elite youth soccer players. International Journal of Environmental Research and Public Health, 17(3). doi.org/10.3390/ijerph17030729
ISSN: 2014-0983
Received: January 23, 2024
Accepted: March 22, 2024
Published: October 1, 2024
Editor: © Generalitat de Catalunya Departament de la Presidència Institut Nacional d’Educació Física de Catalunya (INEFC)
© Copyright Generalitat de Catalunya (INEFC). This article is available from url https://www.revista-apunts.com/. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/deed.en


